光催化烯烃杂二官能化反应中醇类磺酸盐的活化
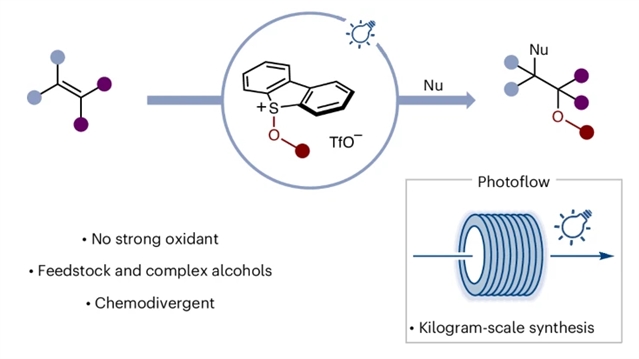
近日,英国曼彻斯特大学Procter, David J.团队报道了光催化烯烃杂二官能化反应中醇类磺酸盐的活化。相关论文发表在2025年11月26日出版的《自然-化学》杂志上。
1,2-二醇和1,2-氨基醇结构广泛存在于生物活性天然产物、药物及农用化学品中。这类备受追捧的亚结构理想情况下可通过醇类对烯烃C=C键的直接加成构建,而醇类和烯烃均为化学合成中常见的底物。然而,直接偶联仅在其中一类底物经衍生化处理呈现缺电子特性时才可能实现;此类方法通常需要当量强氧化剂且缺乏普适性。
研究组描述了一种简便方法:在光催化条件下,无论是简单还是复杂醇类均可转化为相应的烷氧基自由基(经烷氧基硫酸盐中间体),进而与烯烃反应生成1,2-二醇和1,2-氨基醇衍生物。该方法可通过光流动系统轻松实现从实验室到工业公斤级生产的转化。光谱分析和对照实验已用于阐明其作用机制。
附:英文原文
Title: Activation of alcohols as sulfonium salts in the photocatalytic hetero-difunctionalization of alkenes
Author: Zhao, Huaibo, Filippini, Dario, Chen, Yiding, Gallego-Gamo, Albert, Natrajan, Louise S., Pantaine, Loc R. E., Romano, Ciro, Procter, David J.
Issue&Volume: 2025-11-26
Abstract: Motifs related to 1,2-diols and 1,2-amino alcohols are found widely in bioactive natural products, drugs and agrochemicals. These highly sought-after substructures would ideally be constructed by the direct addition of alcohols to the C=C bond of alkenes, both common substrate classes in chemical synthesis. However, their direct union is only possible if one of the pair can be rendered electron-deficient through derivatization; such approaches typically require stoichiometric amounts of strong oxidants and often lack generality. Here we describe a straightforward process in which both simple and complex alcohols can be converted under photocatalytic conditions to the corresponding alkoxy radicals—via the formation of alkoxy sulfonium salts—that react with alkenes en route to 1,2-diol and 1,2-amino-alcohol derivatives. The method can be easily adapted from laboratory to industrial, kilogram scale using a photoflow system. Spectroscopic analysis and control experiments have been used to probe the underpinning mechanism.
DOI: 10.1038/s41557-025-02003-7
Source: https://www.nature.com/articles/s41557-025-02003-7
